AQA GCSE Chemistry Periodic Table and Atomic Structure Here are the best resources to pass Periodic Table and Atomic Structure at AQA Find Periodic Table and Atomic Structure study guides, notes, assignments, and much more We also have lots of notes, study guides, and study notes available for GCSE Chemistry at AQA22 recall the positions of metals and nonmetals in the Periodic Table From left to right across a period there is a gradual change from metal to nonmetal elements For example, in Period 3, sodium, magnesium and aluminium are metals They allThe Periodic Table is split with metals all found on the left side of the table, and non metals found on the right Metals have either 1, 2 or 3 electrons on their outermost shell, so want to lose electrons to form positive ions ( cations ) Nonmetals that have either 5, 6 or 7 electrons on their outermost shell want to gain electrons to form

Aqa Gcse Periodic Table And Atomic Structure Higher Assessment Pack
Gcse periodic table edexcel
Gcse periodic table edexcel-Find my revision workbook here https//wwwfreesciencelessonscouk/workbooks/shop/Image creditsDobereiner "https//commonswikimediaorg/wiki/FileJohann_4 He Helium 2 7 Li Lithium 3 9 Be Beryllium 4 11 B Boron 5 12 C Carbon 6 14 N Nitrogen 7 16 O Oxygen 8 19 F Fluorine 9 Ne Neon 10 23 Na Sodium 11 24 Mg Magnesium 12 27 Al Aluminium 13 28 Si Silicon 14 31 P Phosphorus 15 32 S Sulfur 16 355 Cl Chlorine 17 40 Ar Argon 18 39 K Potassium 19 40 Ca Calcium 45 Sc Scandium 21 48 Ti Titanium 22 51 V Vanadium 23 52 Cr



Fulston Manor School Periodic Table
The Periodic Table of Elements (for O Level Chemistry 5070) TaughtWarecom Prescribed by CIE for examination in 16 lithium sodium 23 potassium 39 rubidium 85 caeslum 133 francium Be beryllium 12 Mg magnesium 24 calcium 40 strontium barium 137 radium scandium 45 yttrium 5771 lanthanoids actinoids lanthanumDiscover how the periodic table was developed by John Newlands and Dmitri Mendeleev with BBC Bitesize GCSE Chemistry30 seconds Report an issue Q In the modern periodic table elements are arranged by answer choices atomic mass atomic number valence electrons number of isotopes
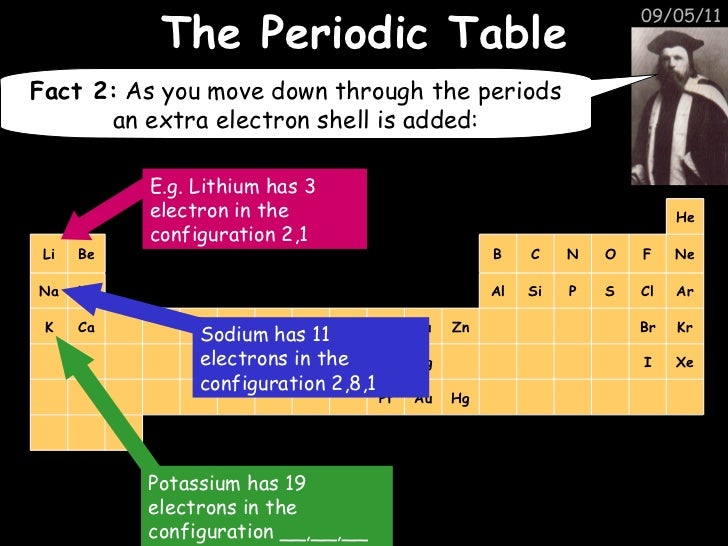
There is a link between the electronic configuration of the elements and their position on the Periodic tableAQA GCSE Combined Science Trilogy Chemistry exam revision with questions & model answers for The Periodic Table Made by expert teachersLearn who made the periodic table, why he arranged the elements this way and about the important groups you need to know
C 22 Electronic structure and the periodic table AQA GCSE Chemistry C2 The Periodic Table Kerboodle Answers Page No 25 1a Periodic means that the elements in the periodic table shows patterns in electronic configuration, physical and chemical properties and repeat itself with increasing atomic numberThe periodic table is used a lot in chemistry It has tons of information on it and if you know how to use it properly, it can really give you the edge when it comes to getting marks in the exam Here are some handy tips on the importance of the periodic table NEW 16 GCSE Chemistry The Periodic Table full lesson Lesson Resource Pack, contains Brand new Powerpoint lesson Blank periodic table to colour in Diagram of an element to label A worksheet about subatomic particles A homework sheet from past exam questions relating to periodic table If this resource is popular I will upload more



Gravesend Tutorial Service Periodic Table Groups



Periodic Table Of The Elements Gcse Chemistry Revision Centre
Mark scheme for questions on The Periodic Table 1 from Edexcel GCSE Chemistry past papers Home / Edexcel GCSE Chemistry / Topic Questions / The Periodic Table 1 Mark Scheme The Periodic Table 1 Mark Scheme Sumon Ahmed T 21ThePeriodicTableMSGCSEEDEXCELCHEMISTRYThe Periodic Table shows Metals (in brown) and NonMetals (in blue) Transition Metals (in pink) have no group number See How to use the Periodic Table or click on one of the elements above, or visit chemical symbols Printable Periodic Table Home GCSE Chemistry IndexPeriodic Table The Royal Society of Chemistry's interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic table Click the tabs at the top to explore each section Use the buttons above to change your view of the periodic table and view Murray Robertson's stunning Visual Elements artwork



The Periodic Table Revision Notes Igcse Chemistry Oxnotes Gcse Revision



Gcse Chemistry The Periodic Table Aqa 9 1 Youtube
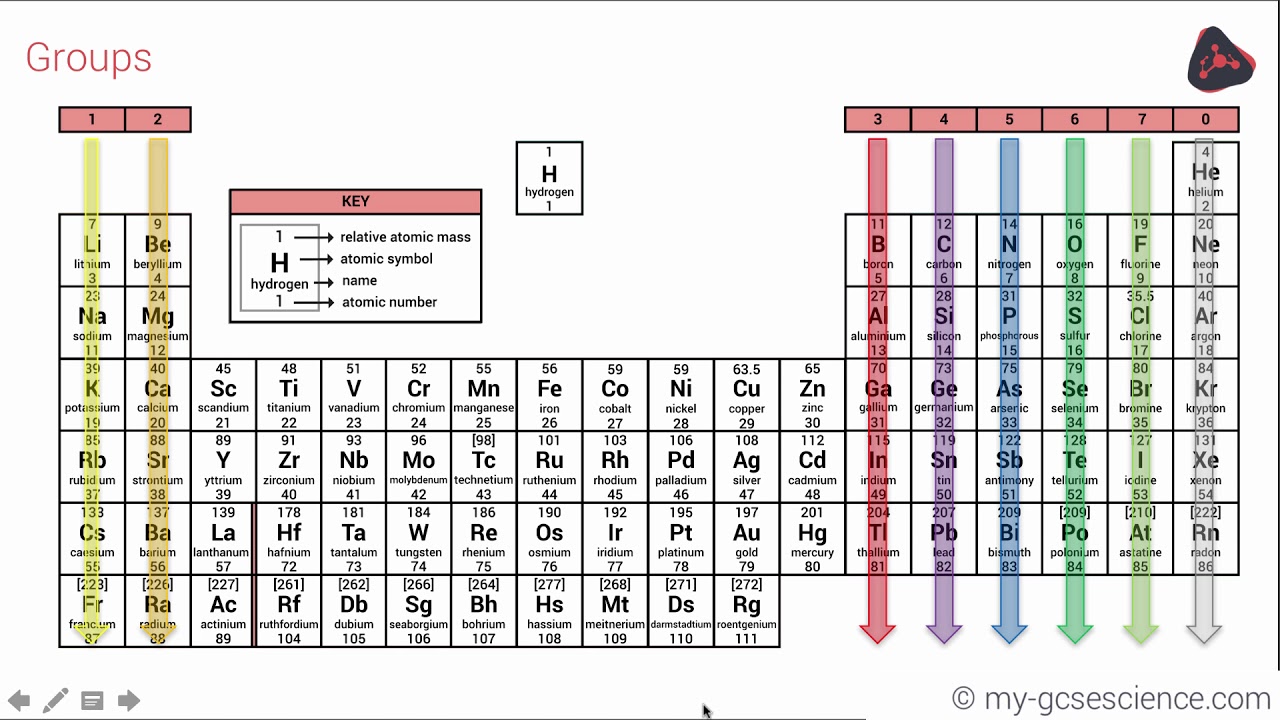
This video relates to the AQA (91) GCSE Chemistry specification which will be examined for the first time in 18 Check out more of our AQA GCSE science viPeriodic table Elements are arranged on the Periodic Table in order of increasing atomic number, where each element has one proton more than the element preceding it The table is arranged in vertical columns called Groups numbered 1 – 8 and in rows called Periods Eg Group 4 elements have atoms with 4 electrons in the outermost shell, Group New Available Printable Gcse Periodic Table The periodic tables were actually created so that scientists could very easily determine the atomic weights of numerous elements In doing so, they could actually prepare the periodic structures for all of the elements, that happen to be needed for an understanding in the functions of your atom




Placing Elements In Order S Cool The Revision Website



The Periodic Table Gcse The Science Hive
Take a quiz on this topic to find out if you know the content!The Periodic Table The History of the Periodic Table The periodic table came about through attempts by people to group elements according to their chemical properties It is called periodic because similar chemical properties of the elements were found to occur at regular intervals What are Dobereiner's Triads?The Periodic Table History of The Periodic Table GCSE ChemistryIn this video, we look at Dmitri Mendeleev's Periodic Table and how it is organised with m




Gcse Chemistry The Basics Lessons By Beatrix Huissoon Scoodle




Gcse Periodic Table As Credited To Dmitri Mendeleev Periodic Table Gcse Chemistry Dmitri Mendeleev
Dobereiner (19) found that some groups of three elementsStart studying Chemistry AQA GCSE Atomic Structure and the Periodic Table Learn vocabulary, terms, and more with flashcards, games, and other study toolsPeriodic Table GCSE Fill The Blanks Chemistry Teaching is a free printable for you This printable was uploaded at by tamble in Periodic Table Here is the Periodic Table GCSE Fill The Blanks Chemistry Teaching you get from Printable Gcse Periodic Table




Periodic Table Year 9 S3 Chemistry Home Learning With c Bitesize c Bitesize



The Periodic Table Quiz Questions Footprints Science Gcse Science Animations And Quizzes
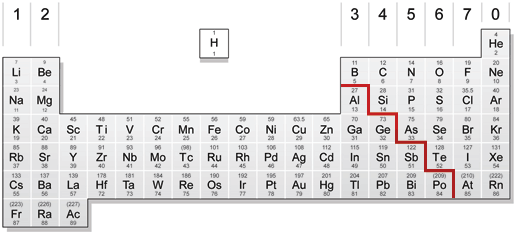
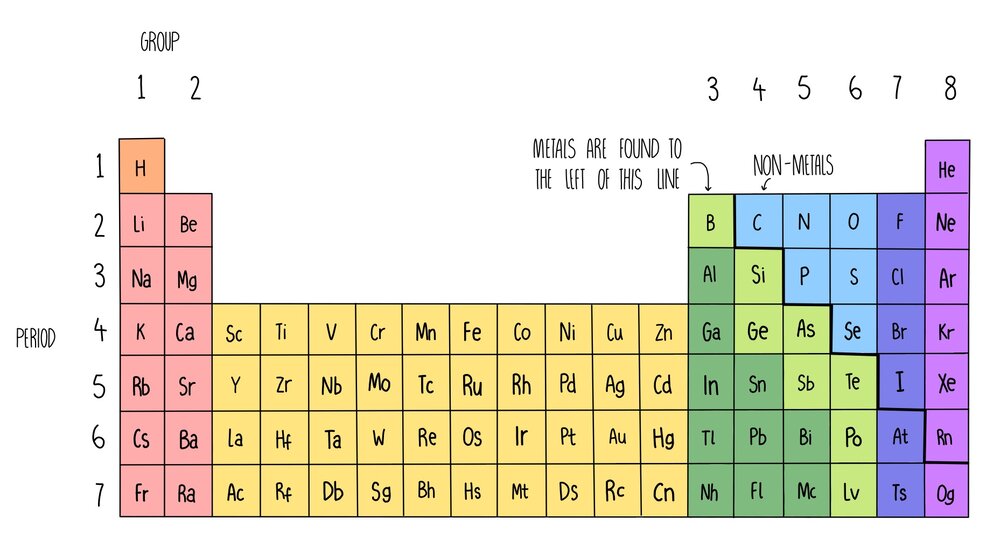
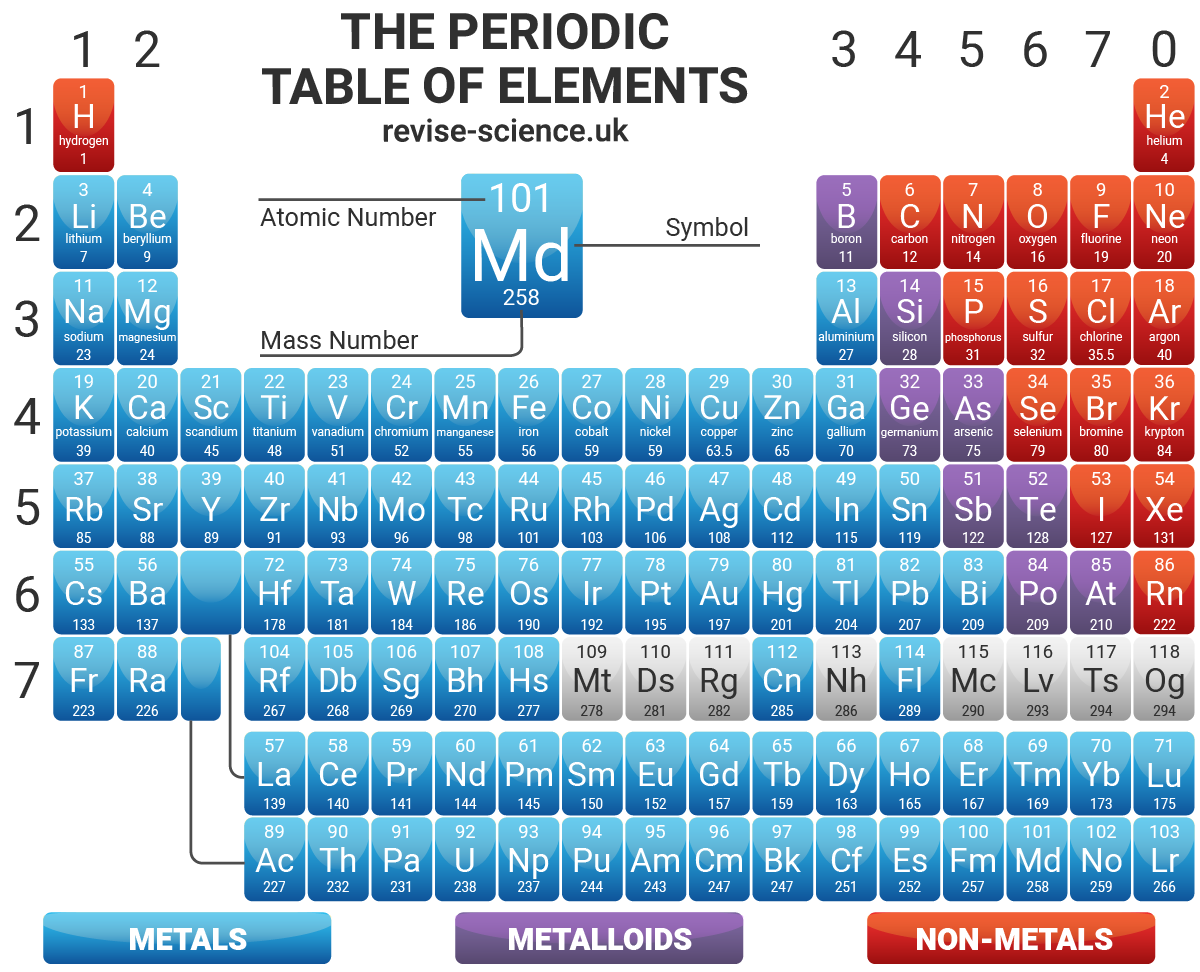
The periodic table below is based on the ones used by the different examination boards The group numbers 1 to 0 (the top ones) are used in most GCSE courses The group numbers 1 to 18 were recommended by IUPAC in 19 At the moment these are only used in OCR courses There is a summary at the bottom of the page It shows the differencesEnjoy the videos and music you love, upload original content, and share it all with friends, family, and the world onThe zigzag line in this diagram separates the metals, on the left, from the nonmetals, on the right Hydrogen is a nonmetal but it is often put in the middle Most elements are metals, rather




C1 1 Fundamental Ideas In Chemistry The Periodic Table Cgp Books Gcse Science Chemistry Lessons Chemistry Education




Colouring Labelling The Periodic Table Teaching Resources
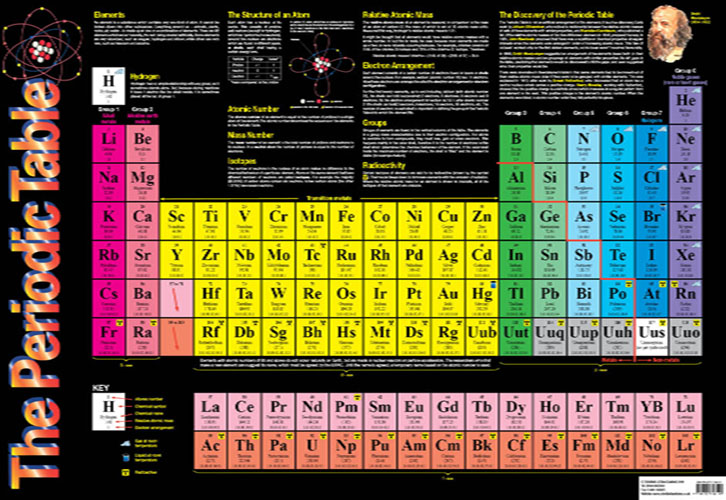
Specification Point 113 Describe how Mendeleev arranged the elements known at that time, in a periodic table by using properties of these elements and their compounds In 1869 Dmitri Mendeleev created his first draft of the Periodic Table He started by writing down the names of the 50 elements known at that time on pieces of paper andThere are seven horizontal periods (rows) in the periodic table The period number is also the number of occupied energy shells in the atoms of the elements in the period So if an element has 3 valence electrons, it will be in group 3 And if it has 4 occupied energy shells, it will be in period 4 We have two types of elements in the periodicElectronic configuration and the Periodic table The electronic configuration is the arrangement of electrons into shells for an atom (eg the electronic configuration of carbon is 2, 4);



19 The Year Of The Periodic Table Rick Anderson




New Gcse Separate Sciences Ocr Gateway Sb Page 316
View periodic_answerspdf from CHE MISC at IoBM GCSE CHEMISTRY THE PERIODIC TABLE ANSWERS AND MARK SCHEMES QUESTIONSHEET 1 noble/inert/rare gases 1 (ii) two out of helium, neon, argon, krypton,Mendeleev made an early periodic table In the modern periodic table, elements are in order of atomic number in periods and groups Electronic structuresStart studying gcse chemistry periodic table Learn vocabulary, terms, and more with flashcards, games, and other study tools




The Periodic Table Chemistry Gcse Revision



Gcse Periodic Table Revisechemistry Uk
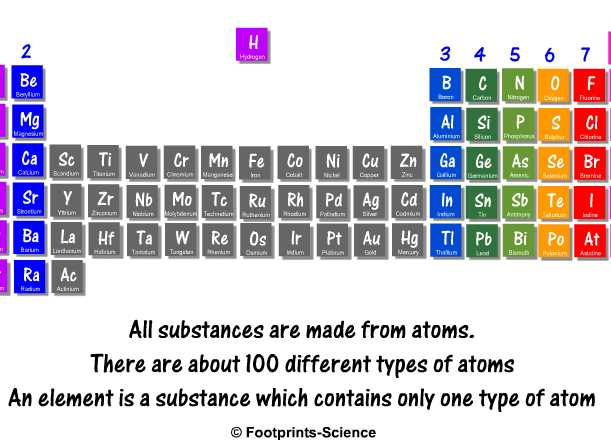
Start studying GCSE Chemistry Topic 1 Atomic Structure and the Periodic table TR Learn vocabulary, terms, and more with flashcards, games, and other study toolsPeriodic Table – A table that shows arrangement of all the known elements in the order of increasing atomic number The table is organised into periods and groups Metals Elements found to the left of the periodic table which are soft, shinny, conductors malleable and ductile eg Group 1;Home > GCSE Chemistry > The Periodic Table & Chemical Patterns Development of the Periodic Table Some 0 years ago scientists were discovering lots of new elements The challenge they faced at the time was the difficulty in finding any links between the different elements It must be remembered this was well before the atomic structure of




Edexcel Gcse Chemistry Topic 1 The Periodic Table Diagram Quizlet




Atomic Structure Knowledge Organiser Aqa Science Beyond
AQA GCSE C2 The Periodic Table Selection of Exam Questions Head of Science and examiner for AQA and OCR Selection of exam questions from AQA that are useful when teaching the C2 topic in the new AQA Trilogy and Chemistry specificationThe horizontal row on the periodic table is called a Periodic Table GCSE DRAFT 8th 10th grade 0 times Chemistry 0% average accuracy 3 minutes ago ctuttlebee_ 0 Save Edit Edit Periodic Table GCSE DRAFT 3 minutes ago by ctuttlebee_ Played 0 times 0 8thThe GCSE Chemistry periodic table of elements is something which you have to get to know pretty well for your exam From Mendeleev and the history of the periodic table to the alkali metals and the halogens, the periodic table contains many subtopics within it, each of which is very important for the Edexcel, OCR and AQA GCSE Chemistry exams



2




Aqa Gcse Chemistry Periodic Table Structure Theory Questions Answers
Topic 6 – Groups in the periodic table GCSE study in the sciences provides the foundation for understanding the material world Scientific understanding is changing our lives and is vital to the world's future prosperity All students should learn essential aspects of the knowledge, methods, processes and uses ofGroup 2 and group 3 elementsPass My Exams Easy Exam Revision Notes For GSCE Chemistry is a free printable for you This printable was uploaded at by tamble in Periodic Table This is the Pass My Exams Easy Exam Revision Notes For GSCE Chemistry you can get from Group 1 Periodic Table which you are able to download or print for free




Arranging The Elements Aqa Gcse Combined Chemistry Revision Notes




Printable Periodic Tables Pdf In 21 Periodic Table How To Memorize Things Problem Solving Worksheet
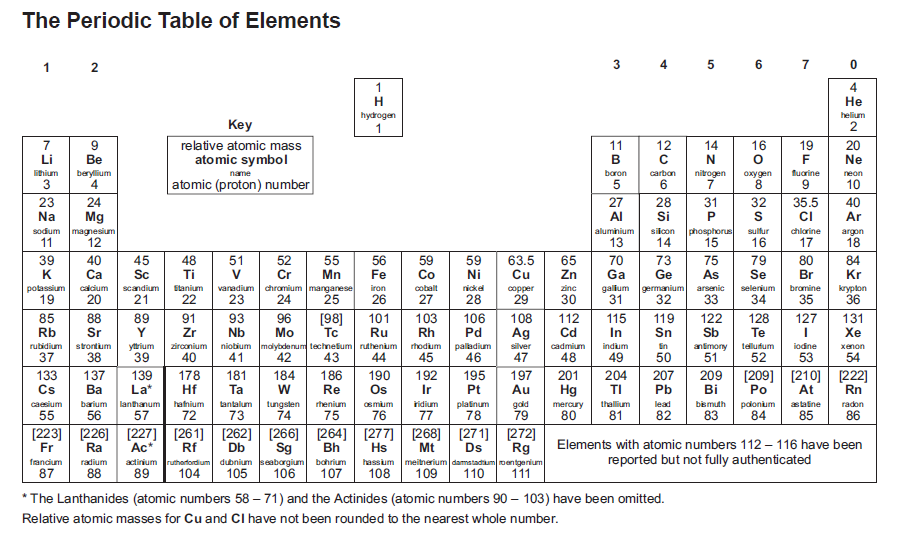
Pass My Exams Easy Exam Revision Notes For GSCE Chemistry is a free printable for you This printable was uploaded at by tamble in Periodic Table Here is the Pass My Exams Easy Exam Revision Notes For GSCE Chemistry you get from Periodic Table Group 1 which you can download for freeThe Periodic Table of Elements 7 Li lithium 3 23 Na sodium 11 39 K potassium 19 85 Rb rubidium 37 133 Cs caesium 55 223 Fr 87 * The Lanthanides (atomic numbers 58 – 71) and the Actinides (atomic numbers 90 – 103) have been omitted Relative atomic masses for Cu and Cl have not been rounded to the nearest whole number francium 12 Be Key 9 beryllium 4 relative atomic mass > GCSE Revision Notes > GCSE Chemistry > Periodic Table of the Elements Periodic Table of the Elements by revisioncentre 19 April 19 April GCSE Chemistry (Elements highlighted in a red font are important elements which you should really know about) Search for Shop Online



Chemistry Gcse Ks Learning



Gcse C4 Chemical Patterns Bonding Periodic Table Revision
GCSE Periodic Table As Credited To Dmitri Mendeleev is a free printable for you This printable was uploaded at by tamble in Periodic Table This is the GCSE Periodic Table As Credited To Dmitri Mendeleev you get from Printable Gcse Periodic Table which you are able to download or print for free



Periodic Table Posters At Schofield And Sims




Gcse The Periodic Table By Anandjsingh Issuu



Wallace S Pictures Periodic Table




Gcse Periodic Table Edexcel
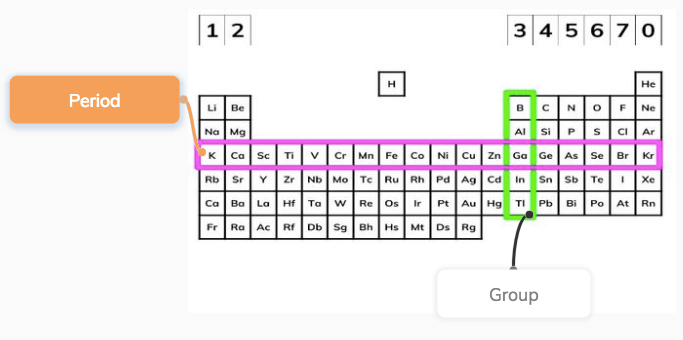



What Is The Periodic Table Definition From Seneca Learning



Fulston Manor School Periodic Table



Periodic Table Revision Cards Gcse Aqa Trilogy Teachit Science



Www Alunschool Co Uk Pages Parentsandstudents Exams Year10 Revision Chemistry year 10 revision Pdf



3



Periodic Table Of Elements Poster Ks3 Chemistry Beyond



Periodic Table Gcse Science Marked By Teachers Com




Stickers Magic Periodic Table Poster Wall Sticker With Elements Home School Science Educational Wall Chart Ks3 Ks4 Gcse Chemistry Student Teacher Usv 023 Amazon Co Uk Stationery Office Supplies




Large Periodic Elements Table Poster Wall Chart For Kids Gcse A Level 21 Ebay




Gcse Chemistry Mind Map Group 0 Periodic Table Webschool Org Uk




Gcse Periodic Table Edexcel
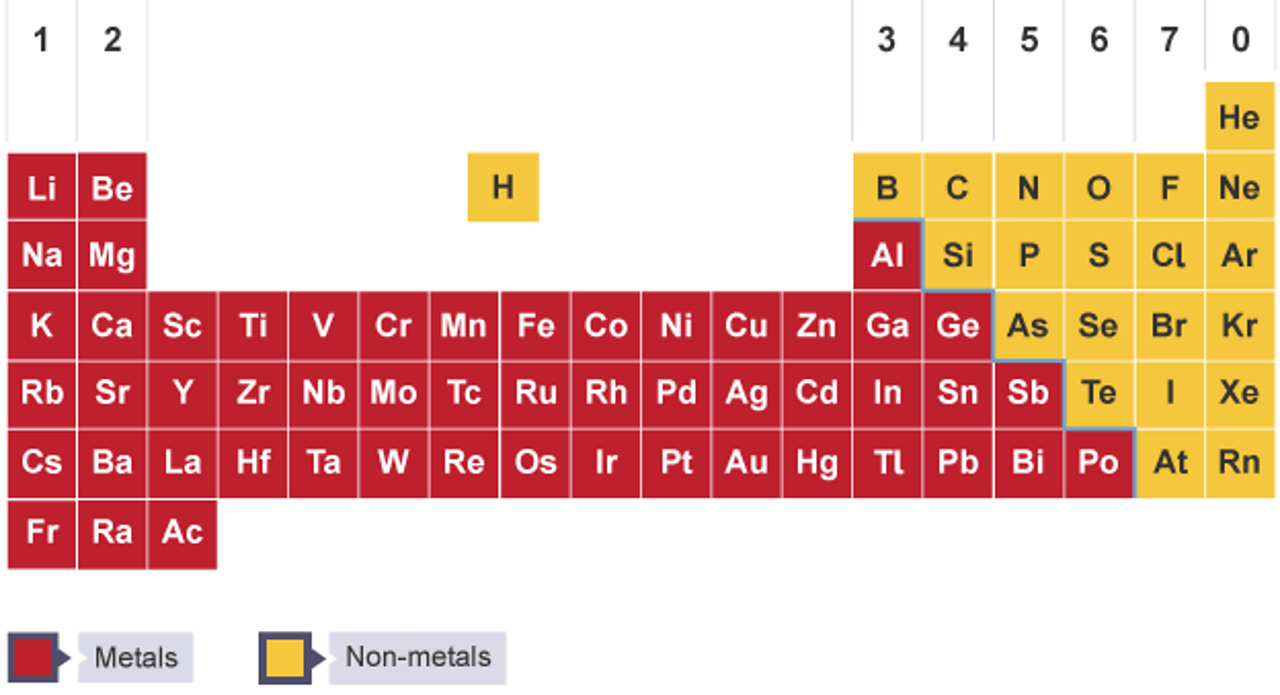



The Periodic Table The Periodic Table Ks3 Chemistry Revision c Bitesize




Aqa Gcse Atomic Structure And Periodic Table Part 2 Ppt Download



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry
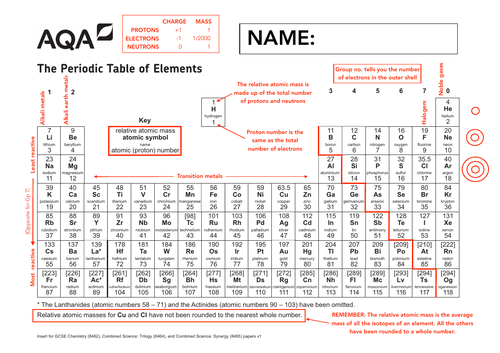



Annotated Periodic Table Aqa Chemistry Teaching Resources




Gcse C2 Chemistry Periodic Table



Science Ogt Periodic Table Of The Elements




What Are Isotopes How Do They Affect Ram Chemistry Made Simple




19 The Year Of The Periodic Table Rick Anderson



Chemistry Aqa Further Gcse Revision Cards In Gcse Chemistry
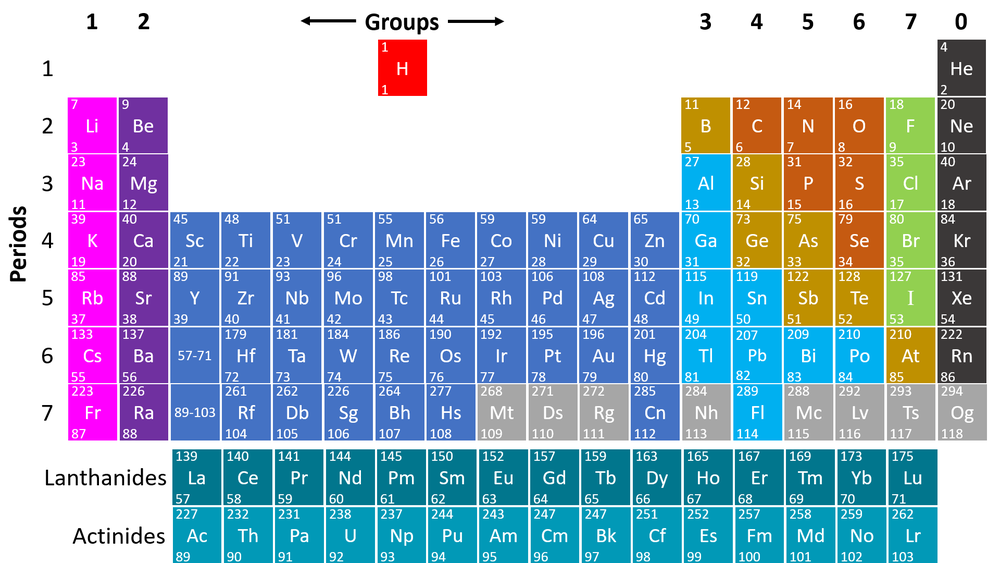



Periodic Table Key Stage Wiki
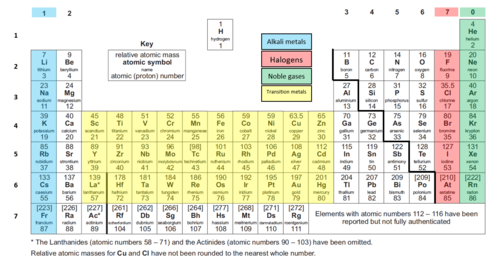



Aqa Periodic Table Clearly Labelled And Colour Coded Version Teaching Resources




Interactive Periodic Table By U Dev Kr Gcse



Gcse Chemistry Introductory Unit Page




Periodic Trends Cie Igcse Chemistry Revision Notes




Understanding The Trends Of The Periodic Table Gcse 9 1




Large A0 Periodic Table Poster Wall Chart Suitable For Gcse A Level 19 Ebay
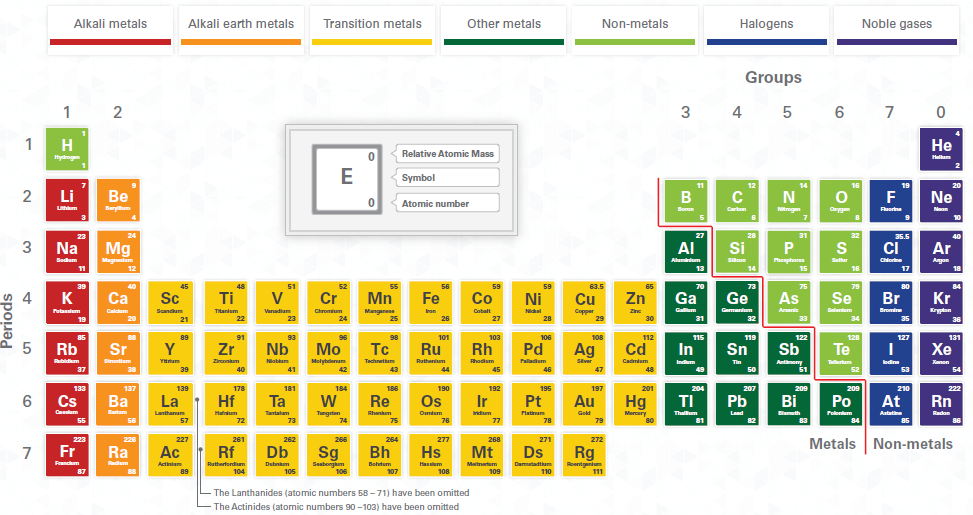



19 The Year Of The Periodic Table Rick Anderson
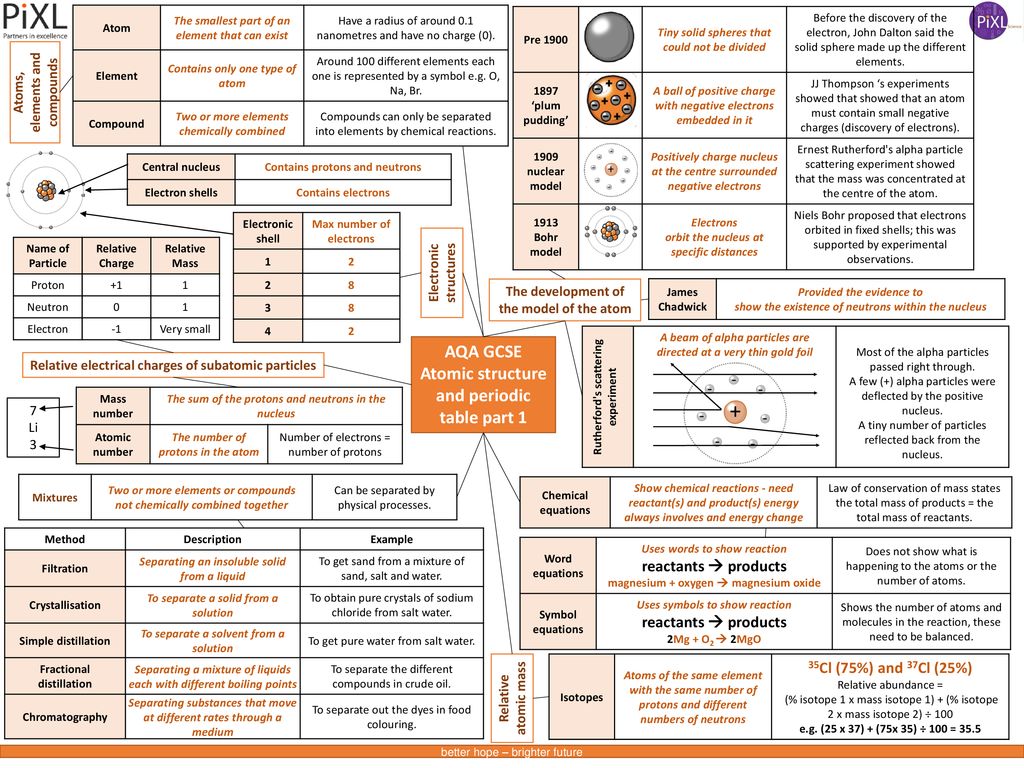



Aqa Gcse Atomic Structure And Periodic Table Part 1 Ppt Download



1




Aqa Gcse Periodic Table And Atomic Structure Higher Assessment Pack



Pmt Physicsandmathstutor Com Download Chemistry Gcse Past Papers Wjec Wales Unit 1f Specimen qp unit 1 F wjec chemistry gcse Pdf




C1 1 The Periodic Table Secondary Science 4 All




Gcse Chemistry Notes For Groups In The Periodic Table Grade Etsy




Stationery School Equipment Large Periodic Table Poster 19 A1 Wall Chart Suitable For Gcse A Level Home Furniture Diy




History Of The Periodic Table Aqa Gcse Chemistry Revision Notes




Large Periodic Table Poster A1 Wall Chart Suitable For Gcse A Level 19 59 4 84 1 Cm Amazon Co Uk Stationery Office Supplies




Aqa Gcse 9 1 Chemistry Quiz On Topic 1 Atomic Structure And The Periodic Table




Chemistrygcse Co Uk Simple Clear And Well Explained




Gcse Periodic Table Introduction Worksheet Questions On Basic Ideas Of Its Structure Igcse Ks4 Science Revision Questions
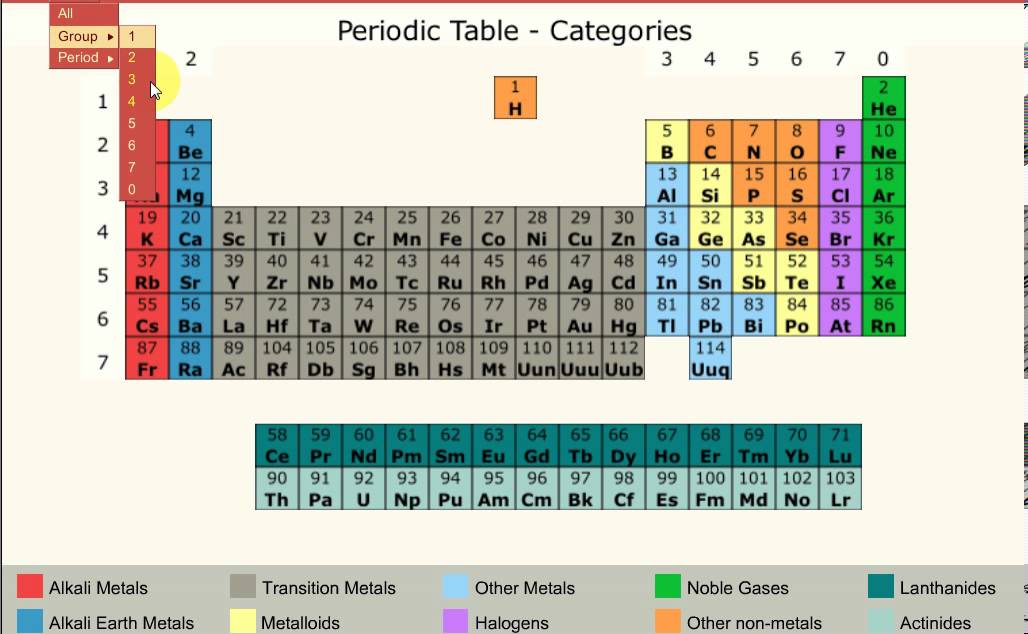



Aqa Gcse Chemistry Unit 1 Fundamental Ideas Pt2 Periodic Table Youtube



Ks4 Gcse Chemistry The Periodic Table Revision Resources For Dyslexics



1 18 Understand How Elements Are Arranged In The Periodic Table In Order Of Atomic Number In Groups And Periods Tutormyself Chemistry




Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Business Industry Science Education Supplies Umoonproductions Com




Savvy Chemist Gcse Ocr Gateway Chemistry C2 2 A C Metals And Non Metals




Group 1 Aqa Gcse Chemistry Revision Notes




Aqa Gcse Chemistry C2 Periodic Table Worksheets Teaching Resources




Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Amazon Co Uk Stationery Office Supplies



Www Ocr Org Uk Images Periodic Table Of The Elements Poster Pdf



1
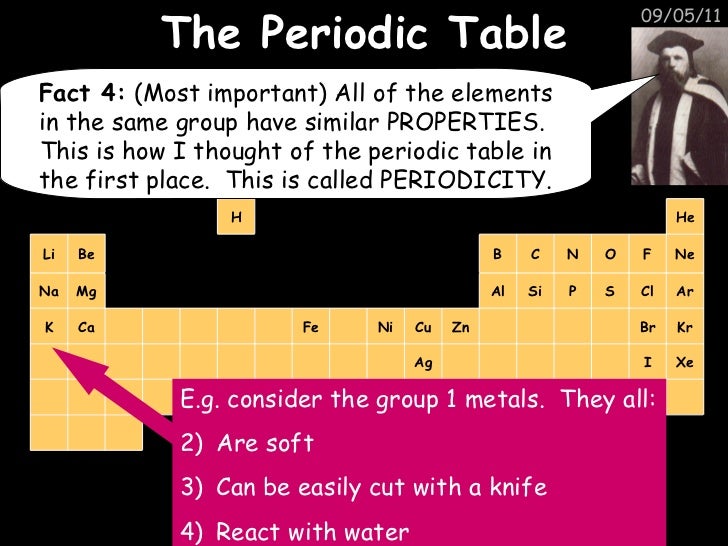



Gcse C4 Chemical Patterns Bonding Periodic Table Revision
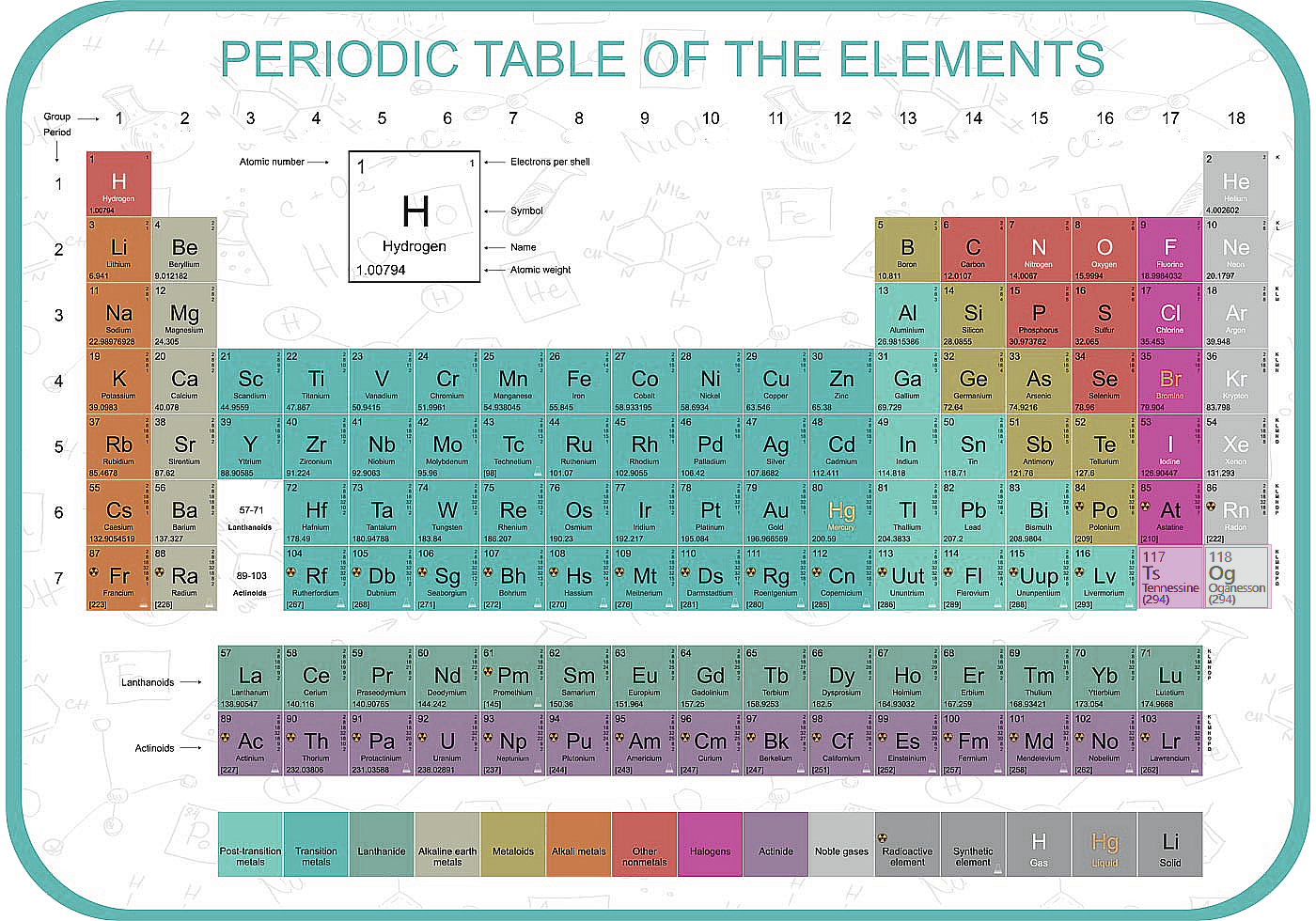



Physics Revision Gcse And A Level Physics Revision Cyberphysics The Revision Website



Http Www Lordwilliams Oxon Sch Uk Force Download Cfm Id 4859




Color Periodic Table With Shells Gcse Periodic Table With Mass And Atomic Numbers Full Size Png Download Seekpng



Pmt Physicsandmathstutor Com Download Chemistry Gcse Notes Aqa 1 Atomic Structure And The Periodic Table Summary 1 2 the periodic table Pdf




Periodic Table Questions Gcse Curriculum Press




Gcse Chemistry Atomic Structure And Bonding Chemactive




New Gcse Add L Science Ocr Gateway Sb Page 152




Gcse Chemistry The Periodic Table Aqa 9 1 Youtube



3




A1 Wall Chart Large Periodic Table Poster Suitable For Gcse A Level 19 Toys Learning School Deshpandefoundationindia Org




C1 1 Atomic Structure And Periodic Table Archives Wahibo Education




History Of The Periodic Table Gcse Chemistry Combined Science Aqa Revision Study Rocket




Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Business Industry Science Education Supplies Umoonproductions Com
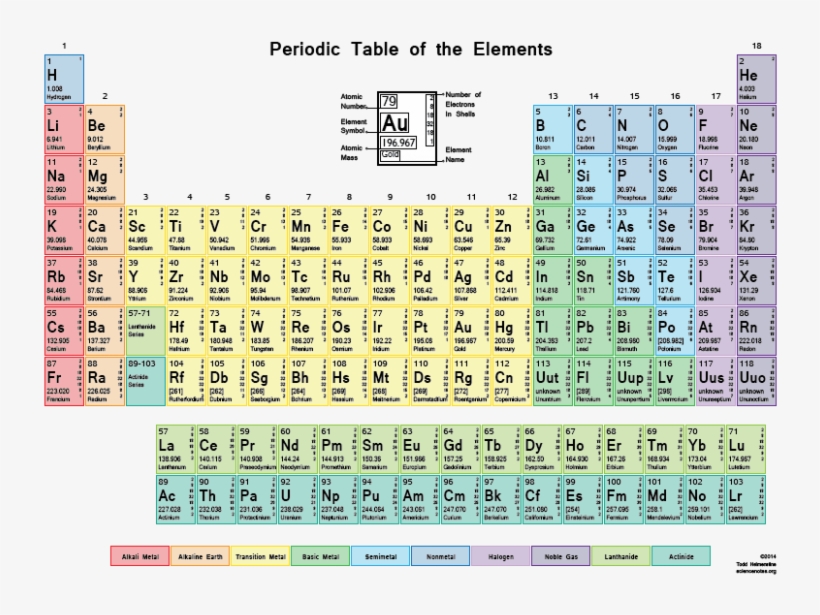



Color Periodic Table With Shells Gcse Periodic Table With Mass And Atomic Numbers Free Transparent Png Download Pngkey




Gcse Modern Periodic Table



Aqa Gcse Chemistry 11 Doc 244 Kb




New 9 1 Aqa Gcse Chemistry Paper 1 The Periodic Table Complete Revision Summary Expert Guidance By Mahima Laroyia



Www Rewardinglearning Org Uk Common Includes Microsite Doc Link Aspx Docid 1




Buy Stickers Magic Periodic Table Poster Display With Elements Home School Science Educational Wall Chart Wall Sticker Ks3 Ks4 Gcse Chemistry Student Teacher Usv 024 Online In Uk B08b4xrg7c




A1 Wall Chart Large Periodic Table Poster Suitable For Gcse A Level 19 Toys Learning School Deshpandefoundationindia Org




Periodic Table Gcse Periodic Table Quiz Quizizz




Aqa Gcse Atomic Structure And Periodic Table Part 1 19 03 05آ Atomic Structure And Periodic Table Pdf Document
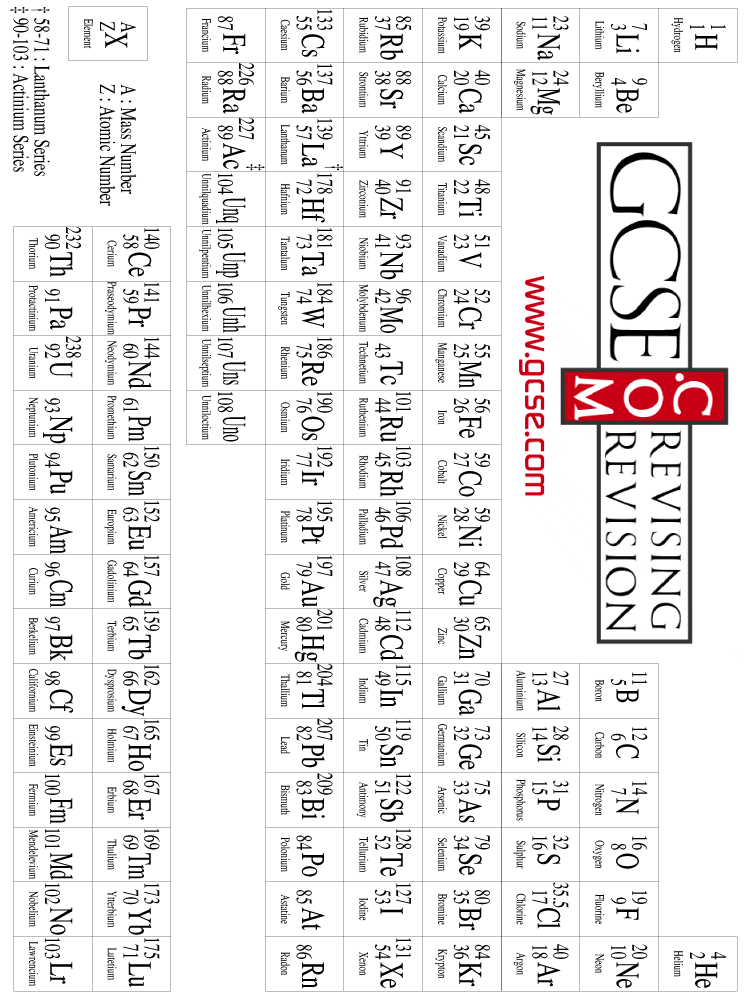



Gcse Com Downloads




Gcse O Level Periodicity The Periodic Table And Its Trends Youtube




Exam Style Questions S Cool The Revision Website


